Atomic Structure & Chemical Bonds
Two major particles are found in the nucleus of most atoms. Protons (have weight and a positive charge) and Neutrons (equal weight to a proton and no charge). Outside the nucleus in shells (subdivided into orbitals) are electrons (no weight and a negative charge)
Usually Protons = neutrons = electrons and since protons have a positive charge and electrons have an equal but opposite negative charge atoms are neutral.
Isotopes in most cases are when the proton No. is not identical to the neutron number (exceptions include Hydrogen). Usually the neutron No. is greater than the proton no.
For a given element the proton number is constant and fixed, if there are isotopes the neutron number is variable.
Atomic Number is the number of protons in an atom
Mass number is the number of protons + neutrons ( usually twice the atomic number)
Atomic weight is the average of mass numbers of all isotopes of an atom taking into account their relative proportions in a sample (see examples below)
Element Atomic No.(protons) Mass No.(protons +neutrons) Atomic weight Electrons
Hydrogen 1 1 1
1 2 (1 neutron) 1
1 3 (2 neutrons) 1.008 1
Helium 2 4 4 2
Carbon 6 12 6
6 13 6
6 14 12.01 6
Stability and therefore reactivity and bond formation is a result of the organization of electrons in the orbitals surrounding the nucleus. See Fig 1. below for the organization of some common atoms. The number of electrons required to be added to or lost from the outermost shells dictate the number of bonds that must be formed to make the atoms stable.
Fig.1 See diagrams in your notes.
Notice, the number of shells in the atoms are variable as are the number of electrons in the shells. The unstable and therefore reactive atoms are hydrogen, carbon, oxygen, sodium and chlorine. The stable atom and thus unreactive atom is helium. Given that the inner most shell of all atoms can only contain two electrons and subsequent shell a maximum of eight electrons you should be able to see that atoms that do not have the maximum number of electrons in the outer most orbital are the ones that are unstable and reactive and also the number of bonds required to be formed to make the atoms stable. Hydrogen-1, carbon-4, oxygen-2, sodium-1 and chlorine-1.
Bonds: Three major types of bond are associated with biology, Ionic, covalent and hydrogen. Ionic are the least important but have intermediate strength. Hydrogen are the weakest but of import while covalent are the strongest and of most import in biology.
Ionic bonds:
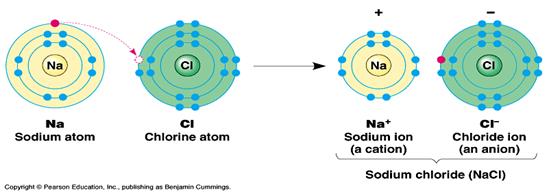
Ionic bonds are formed between ionized atoms (charged). When atoms lose electrons from the outermost shell to become stable they become positively charged (Cations) due to an excess of protons in the nucleus. The number of positive charges ( and therefore potential bonds) is dependant on the number of electrons lost. If atoms gain electrons in the outer most shell to become stable an excess in negative charge arises dependant on the number of electrons gained (anion). In the case of sodium chloride (fig 2), sodium loses the single electron in the outermost shell thus the remaining outer shell is now full. As a consequence there is a single net positive charge. The chlorine accepts the electron lost to complete its outermost shell and becomes stable with a net negative charge due to the excess of electrons. The exchange of electrons has to be complimentary. Magnesium loses the two electrons in its outer most shell to become stable and thus has two positive charges. Magnesium can therefore ionically bond with two chlorine atoms since each will gain one of the two electrons lost. The ionic bonds are actually electrostatic attractions between the opposite charges of the opposite ions. The bond does not have to permanent because the charged atoms are stable.
Fig 2 .
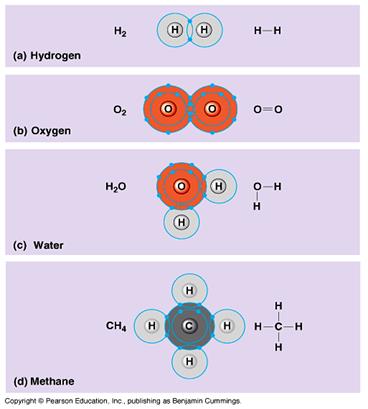
Covalent Bonds:
In the following figure are representations of some simple molecules containing covalent bonds. In all cases the incomplete outer most orbitals of the atoms involved overlap such that pairs of electrons can be shared to complete the outermost orbitals. For example hydrogen atoms have a single orbital containing one electron. To be complete the orbitals require two electrons. By overlapping the orbitals the electrons in each atoms outermost orbital are also found in the orbital of the other atom. Thus each outer orbital now theoretically contains two electrons and is thus stable. The resulting covalent bond is a result of sharing a pair of electrons, one from each atom. The number of covalent bonds formed is dependant on the number of pairs of electrons that need to be shared to complete the outermost orbital. In the example below, the carbon atom in methane has four electrons in the outermost orbital, thus requires four more to be stable. Notice the carbon covalently bonds with four hydrogen atoms, thus sharing the hydrogens four electrons, thus completing the outermost orbital. By the same token each hydrogen atom shares one of the four carbons electrons also becoming stable. In the case of oxygen each atom shares two pairs of electrons to become stable, and therefore forms two covalent (double) bonds.
Hydrogen Bonds:
Polar molecules, such as water molecules and amino acids have a weak, partial negative charge at one region of the molecule (the oxygen atom in water) and a partial positive charge elsewhere (the hydrogen atoms in water). The polarity is usually the result of unequal sharing of electrons (due to significant differences in the mass of nuclei of atoms) during covalent bond formation.
Thus when polar molecules are close together, their positive and negative regions are attracted to the oppositely-charged regions of nearby molecules. The force of attraction, shown here as a dotted line, is called a hydrogen bond. Each water molecule is hydrogen 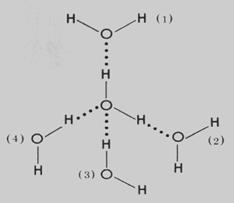 bonded to four others.
bonded to four others.
The hydrogen bonds that form between water molecules account for some of the essential — and unique — properties of water.
Two outcomes of this:
The hydrogen bond is relatively weak. However, when many hydrogen bonds can form between molecules (or parts of the same molecule), the resulting union can be sufficiently strong as to be quite stable.
Macromolecules:
There are three true major macromolecules and thus polymers (carbohydrates, proteins & nucleic acids) and one that is not truly a macromolecule (lipids). While each may have several functions each has at least one unique function.
Carbohydrates- Covalent bonds are the major source of energy for cells when broken.
Proteins- Enzymes, the biological catalysts that regulate the reactions of life.
Nucleic Acids – The storage of Genetic information (enzymes) in DNA, and RNA for
the conversion of the genetic information into Protein.
Lipids- Membranes
All theoretical polymers are made of repeating subunits (monomers) covalently bonded together by a process that usually requires the removal of apart of water from one monomer and a part from the other monomer involved in the covalent bond. The name of the covalent bond formed depends on the monomers involved and the macromolecule formed.
Ex. Monomer A Monomer B
A- O-H + H-O-B
(The italicized atoms are those parts of water to be removed.)
thus, A-O- B + H-O-H
is the resulting reaction, known as a condensation reaction or Dehydration Synthesis.
Carbohydrates.
Saccharide in greek means sugar and is the basis for naming the monomers and complexes of monomers of the carbohydrates.
The monomers of carbohydrates are grouped into classes based on the following carbon, hydrogen and oxygen associations
(CH2O)n where n can have the following values:
3 = triose 4 = tetrose 5 = pentose 6 = hexose 7 = heptose
Triose and tetrose monosaccharides are always organized as a linear chain whereas pentose, hexose and heptose are either organized as linear chains or ring structures, usually ring structures in biological material. While the number of carbon, hydrogen and oxygen atoms in a given class are fixed the specific arrangement is variable. Specific arrangements of atoms give rise to specific monosaccharides within a class. Glucose and fructose are both hexose monosaccharides with different arrangements of atoms (Fig 2b) and therefore different properties.
Carbohydrates can be classified as simple – monosaccharides, short chain- oligosaccharides ( a few monosaccharides covalently bonded together), or complex carbohydrates- polysaccharides (many monosaccharides covalently bonded together). A specific short chain carbohydrate with two monosaccharides covalently bonded together is termed a disaccharide. The formation of the covalent bond to link monosaccharides together requires making the monosaccharides to be bonded unstable. This is accomplished by removing specific functional groups or parts of function groups from the carbon skeleton of the individual monosaccharides to be bonded. An O-H is removed from one monosaccharide and a H from the other. The unstable parts of the monosaccharides then share electrons to generate stability in a covalent bond called a glycosidic bond (Fig 2). Additionally the H and O-H also covalently bond to produce water. This reaction is a condensation reaction or Dehydration synthesis. To break this bond the reverse is required- the addition of water or Hydrolysis.
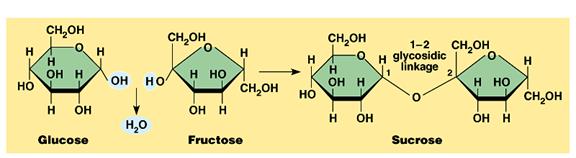
Proteins:
Proteins being polymers are also made of monomers covalently bonded together. In this case the monomers are called Amino Acids of which there are twenty different ones available to construct the proteins
The basic structure of an amino acid is presented below:
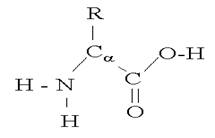
While it is useful to know all structures, minimally the structure of Cysteine (the presence of a sulfhydryl group [-H-S] at the terminus of the R group) should be known because of its importance in the structural organization of proteins. Additionally all the amino acids have a slight negative charge associated with the –C=O (carboxyl) group and a slight positive charge associated with the –N-H (amine) group in the protein as a result of unequal sharing of electrons, and thus have the ability to form hydrogen bonds which again will be of importance in the structural organization of proteins.
The structures of proteins are more complex than carbohydrates in that there are potentially four sequential levels of organization (hierarchy) termed Primary, Secondary, Tertiary and Quaternary structures.
Primary Structure is the linear sequence of Amino acids in a given protein (encoded for in DNA). The specific sequence of amino acids is maintained by the formation of covalent bonds in a similar manner to that in carbohydrates. That is parts of water are removed from the functional groups of adjacent amino acids. –O-H is removed from the –COOH group of the first amino acid in the sequence and H from the NH2 (of the next Amino Acid in the sequence) resulting in the sharing of electrons between the two functional groups of the two amino acids to form a covalent bond termed a Peptide bond (Fig 4). Since the -H and –O-H combine covalently, just as in the formation of a glycosidic bond, the process is known as dehydration synthesis. To break this bond the reverse is required, the addition of water or Hydrolysis.

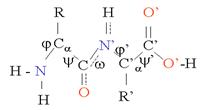
Secondary Structure:
Is the spiraling in space of the primary structure and is maintained using hydrogen bonds between the positive charges on the amine groups and negative charges on the carboxyl groups.
Tertiary structure is the folding of the previous structures and is maintained by covalent bonds called disulfide bridges between the sulfhydryl groups of two adjacent cysteines.
Quaternary structure requires multiple linear sequences of amino acids (organized as above to be hydrogen bonded together.
Lipids:
Lipids are not true macromolecules because the monomers are not covalently bonded together.
Simple lipids are composed of subunits made of fatty acids covalently bonded to a triose sugar – glycerol.
Monoglycerides One fatty acid
Di Two fatty acids
Tri Three fatty acids
Complex lipid subunits are derivatives of diglycerides called phosphodiglycerides because they are composed of two fatty acids, one glycerol and a phosphate group attached to the glycerol. The presence of the phosphate makes the glycerol phosphate polar and therefore hydrophilic whereas the fatty acids are non polar and therefore hydrophobic. It is these properties that are responsible for the organization of the macromolecule into the membrane structure shown below where the circles represent the polar glycerol phosphate and the squiggly lines the fatty acid side chains.
Nucleic Acids:
There are two main functions of nucleic acids, the storage of genetic information (DNA) and the conversion of this information into proteins (via RNA). The monomers of nucleic acids are called nucleotides. In addition to being the monomers of nucleic acids, specific nucleotides and their derivatives can be used for other purposes including storage of chemical energy (ATP –made by phosphorylation of ADP) and as coenzymes in major biochemical reactions (NAD & FAD).
The monomers (nucleotides) are comprised of three components.
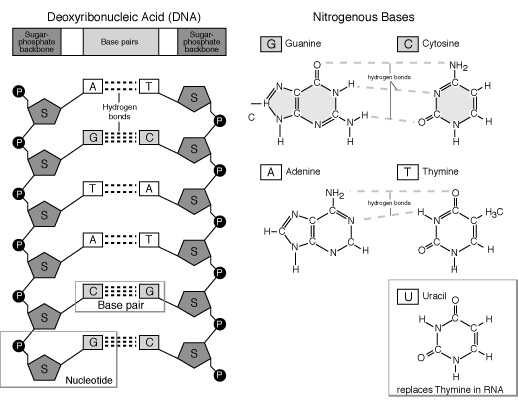
The nitrogen bases are classified as either two ringed purines (Adenine and Guanine) or single ringed pyrimidenes (Thymine, cytosine and Uracil). Both purines (A & G) are found in DNA along with the pyrimidenes Thymine and Cytosine ( T & C). RNA contains both purines (A & G) and the pyrimidenes Uracil and Cytosine ( U & C). [see the figure below].
In both DNA and RNA the nucleotides are covalently bonded together in linear sequences using phosphodiester bonds between the carbon #3 and the phosphate attached to the carbon # 5 of adjacent sugars via of dehydration synthesis.
In DNA molecules there are two linear sequences of nucleotides which are connected using hydrogen bonds between the nitrogen bases of the two strands. This hydrogen bonding is highly specific, the purine Guanine only hydrogen bonds with pyrimidene cytosine and the purine Adenine only hydrogen bonds with pyrimidene thymine. In order to hydrogen bond in such a way one strand of nucleotides has to be upside down and reversed relative to the other [see the figure below]. The resultant strands in the molecule are therefore said to be complimentary and antiparallel. The direction of the strands can be designated therefore as either 5’ to 3’ or 3’ to 5’. If the nucleotide sequence is known in one strand , by definition it is known in the other.
Ex. 5’_____________________3’
A T T C C G A T C G C A
Hydrogen bonds ->
T A AG G C T A G C G T
3’ 5’
Genetic information for the sequence of amino acids in the protein is stored in the sequence of N-bases of the nucleotides in only one of the two strands of DNA (sense strand). Three N-bases (and therefore nucleotides are required to encode for a single amino acid, hence the term Triplet Codon.
RNA is a single stranded polymer of nucleotides covalently bonded similarly to DNA.
However, remember the four bases found are Guanine, Adenine, Cytosine and Uracil.
There are three types of RNA each with its own unique function:-
mRNA – has the amino acid sequence of the protein in the nucleotide sequence.
rRNA- associates with protein to produce a ribosome whose function is to read mRNA and form peptide bonds between the amino acids thus creating the protein.
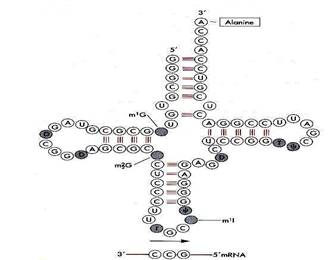
tRNA- is associated with transporting the amino acids required to the site of protein synthesis. tRNA is unusual in that it is folded uniquely so that complimentary areas of nucleotides are antiparallel and thus hydrogen bonds form.
DNA Replication:
This process is for copying the genetic information (DNA-chromosome) so new cells can be created with the same properties as the original cell in which the DNA is replicated. The process involves a series of enzymes (helicases, ligases and polymerases). Each has specialized functions in the process- that is to break the hydrogen bonds and unwind the molecule, fill in any gaps in the new strands to form a continuous strand of nucleotides and attach free nucleotides to the parent or original DNA template strand. For our purposes we will collectively call the enzymes DNA polymerase.
In the process the two complimentary anti-parallel strands unwind, separate and expose the N-bases of the nucleotides. Each strand stays intact and acts as a template for the formation of a new complimentary anti-parallel strand of nucleotides. The new strand is formed according to the base pairing rule A to T and G to C. Each new nucleotide N base forms H bonds with the complimentary base in the old strand and phosphodiester bonds with adjacent nucleotides in the newly synthesizing strand. As the process occurs the strands recoil to form helices.
As a consequence of the mechanism each new molecule of DNA possesses an original strand (old) of nucleotides and a newly synthesized strand. The process is therefore considered “semi conservative”.
Legend:
A simplified representation of a DNA molecule separating to form two new molecules.
To reproduce, a cell must copy and transmit its genetic information (DNA) to all of its progeny.
To do so, DNA replicates, following the process of semiconservative replication. Each strand of the original molecule acts as a template for the synthesis of a new complementary DNA molecule.
The two strands of the double helix are first separated by enzymes. With the assistance of other enzymes, spare parts available inside the cell are bound to the individual strands following the rules of complementary base pairing: adenine (A) to thymine (T) and guanine (G) to cytosine (C).
Two strands of DNA are obtained from one, having produced two daughter molecules which are identical to one another and to the parent molecule.
Protein Syntesis:
Protein Synthesis involves two steps
Transcription: RNA polymerase unwinds the DNA Strands to expose the N bases of the nucleotides to be transcribed to m RNA. The sense strand acts as a template to construct an anti-parallel complimentary mRNA molecule.
Eg
Nonsense DNA 5’ ________________________3’
ATG GTC CCA CGA
mRNA 5’ _______________________3
AUG GUC CCA CGA
RNA polymerase->
Sense DNA 3’ TAC CAG GGT GCT 5’
Translation : mRNA acts as a template to hydrogen bond tRNA molecules to which have specific amino acids attached to them, thus lining the Amino acids up in the correct order to create the protein.
The t RNA molecules have a code that is complimentary and anti-parallel to the triplet codes in the mRNA.
Using the example above
mRNA 5’ _______________________3
AUG GUC CCA CGA
tRNA codes 3’ UAC CAG GGU GCU5’
In order to form the hydrogen bonds between the tRNA and mRNA a ribosome has to be present. It also forms the peptide bond between the Amino acids. Since the ribosome only sits over two triplet codes on the mRNA, to complete the synthesis of protein it needs to move along the mRNA – Translocation.
Source: https://www2.southeastern.edu/Academics/Faculty/kbancroft/macromolecules.doc
Web site to visit: https://www2.southeastern.edu
Author of the text: not indicated on the source document of the above text
If you are the author of the text above and you not agree to share your knowledge for teaching, research, scholarship (for fair use as indicated in the United States copyrigh low) please send us an e-mail and we will remove your text quickly. Fair use is a limitation and exception to the exclusive right granted by copyright law to the author of a creative work. In United States copyright law, fair use is a doctrine that permits limited use of copyrighted material without acquiring permission from the rights holders. Examples of fair use include commentary, search engines, criticism, news reporting, research, teaching, library archiving and scholarship. It provides for the legal, unlicensed citation or incorporation of copyrighted material in another author's work under a four-factor balancing test. (source: http://en.wikipedia.org/wiki/Fair_use)
The information of medicine and health contained in the site are of a general nature and purpose which is purely informative and for this reason may not replace in any case, the council of a doctor or a qualified entity legally to the profession.
The following texts are the property of their respective authors and we thank them for giving us the opportunity to share for free to students, teachers and users of the Web their texts will used only for illustrative educational and scientific purposes only.
All the information in our site are given for nonprofit educational purposes
The information of medicine and health contained in the site are of a general nature and purpose which is purely informative and for this reason may not replace in any case, the council of a doctor or a qualified entity legally to the profession.
www.riassuntini.com