Waves & Radiation Summary Notes
Section 1 – Waves (National 4)
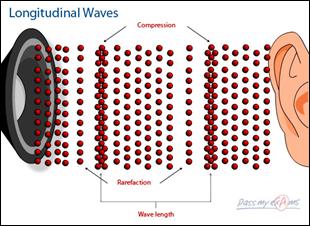
Longitudinal Waves
A longitudinal wave vibrates along the same line as the direction of the wave energy.
Sound energy is a longitudinal wave.
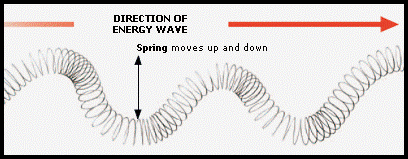
Transverse Waves
In a transverse wave the vibrations making up the wave vibrate at 90º to the direction of the wave’s energy.
All members of the electromagnetic (em) spectrum are transverse waves e.g. radio, microwaves, infra-red, light, ultraviolet, x-rays, gamma rays.
The Speed of Sound (National 4)
Measuring the Speed of Sound
Speed of sound (m/s) =
distance (m)
time on timer (s)
How sound energy is transferred (National 4)
Speed, Distance and Time (sound waves)
speed = distance
time
d = v x t
v = d / t
t = d / v
v
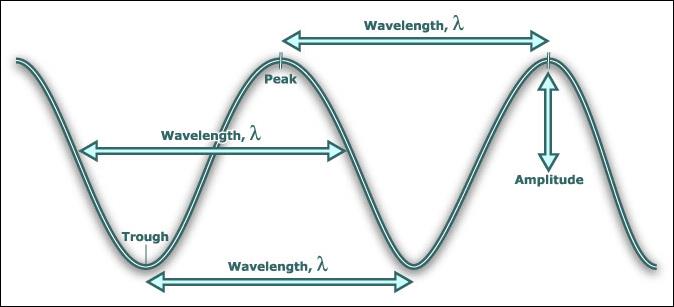
Wave Terms (National 4)
Wavelength, l ● Distance from one point on a wave to the same
(m) point on the next wave.
Amplitude, a ● Size of maximum disturbance from centre (zero)
(m) position. The greater the amplitude, the more
energy in the wave.
Frequency, f ● Number of waves passing a point each second.
(Hz)
Frequency = number of waves
time (seconds)
Wave speed, v ●Distance travelled by the wave in one second.
(m/s)
Speed = distance travelled (m)
time (seconds)
Period, T ●Time taken for one wave to pass a point
(s)
Period = time ¸ number of waves.
![]() or
or ![]()
The Wave Equation (National 4)
Speed = frequency x wavelength
V = f x l
(m/s) (Hz) (m)
Equivalence of v = fl and v= d/t (National 5)
Waves move a distance of one wavelength (l) in a time equal to one period (T).
So when d = l then t = T
However, ![]() =
= ![]()
Therefore if ![]() ,
, ![]()
![]()
Wave Patterns (National 4)
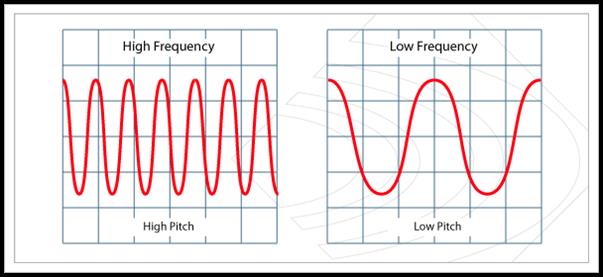
The greater the frequency of a sound wave, the higher the pitch of the sound.
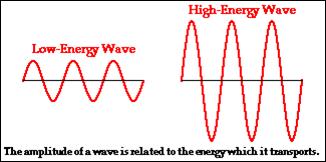
Sound Level (National 4)
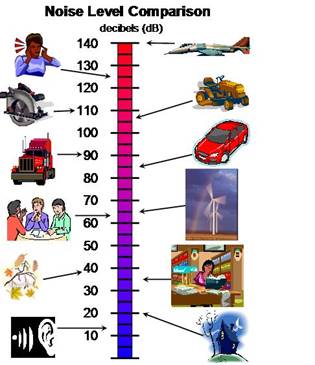
The human ear can be damaged by prolonged exposure to very loud sounds. We can measure the sound intensity using a sound level meter.
The picture below gives typical sound levels in our everyday lives. The least sound intensity our ears can detect is 0 dB.
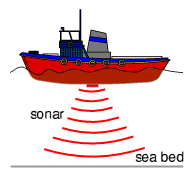
Ultrasound (National 4)
The pictures below show how ultrasound is used in industry, medicine and nature.

Ultrasound Calculations (National 5)
e.g An ultrasonic pulse takes 0.4s to travel to the sea bed and
back to the wave generator. If the speed of sound in water is
1500m/s, calculate the depth of water beneath the ship.
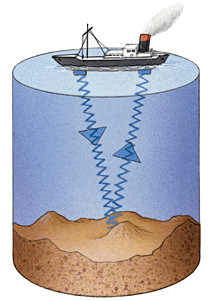
v = 1500m/s d = v x t
t = 0.4s d = 1500 x 0.4
d = ? d = 600m
Distance from ship to the sea bed and back up is 600m.
Therefore, distance from ship to sea bed is 300m.
Depth of water below ship is 300m.
Doppler Effect (National 5)
If the source of a wave gets closer to you, and you are standing still, then you hear the frequency get higher and the wavelength shorter. An example of this is when an express train rushes towards you whilst you stand still on the station platform.
If the source of a wave moves away from you, and you are standing still, then you hear the frequency get lower and the wavelength longer.
Remember the pitch of the sound being produced by the train never changes – you just hear that it does!
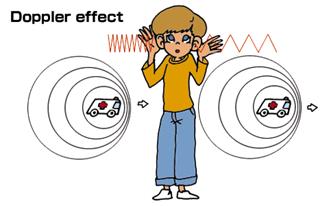
High Pitch
Low Pitch
Diffraction (National 5)
Therefore radio reception is better than television reception in such areas.
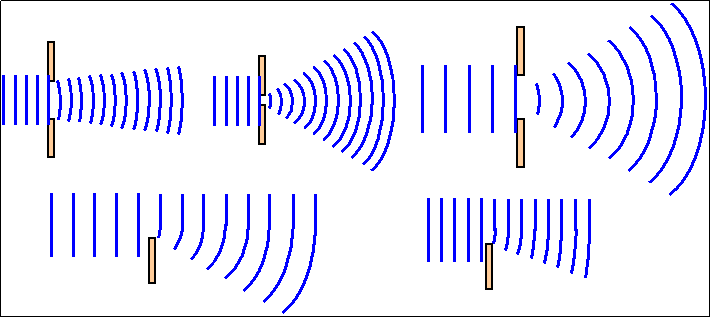
(The distance between the lines represents the wavelength.)
In the diagram above, you can see that the longer wavelength is much better at bending round the obstacle than the shorter wavelength.
LONG WAVELENGTHS DIFFRACT BETTER THAN SHORT WAVELENGTHS.
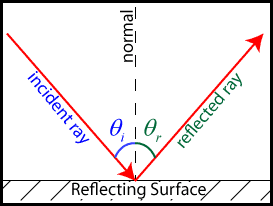
Reflection (National 5)
‘The angle of incidence is equal to the angle of reflection’
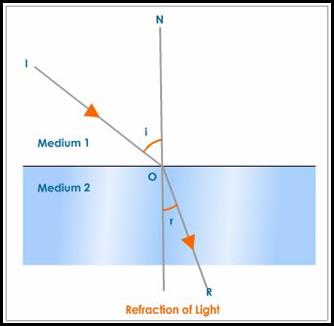
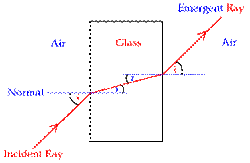
Refraction (National 5)
Refraction occurs when light moves from one medium to another and changes direction due to a change in its speed.
Examples of Refraction (National 5)
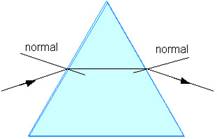
up, and as a result, bends away from the normal.
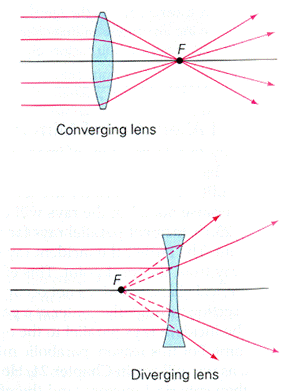
Applications of Refraction (National 5)
Short Sight Long Sight
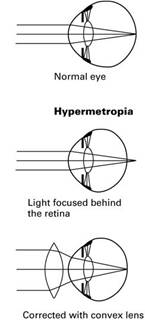
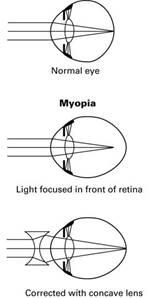
Remember!
Corrected with a concave lens.
Corrected with a convex lens.
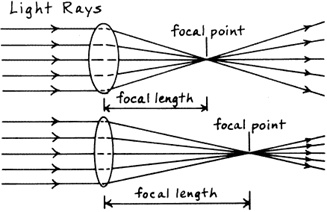
Power of a lens (National 5)
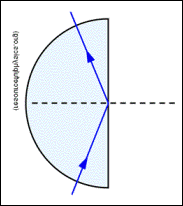
Total Internal Reflection (National 5)
e.g. glass to air, and the angle of incidence in glass gives a refracted angle of 90º in air, then the angle in of incidence in the glass is called the ‘critical angle’
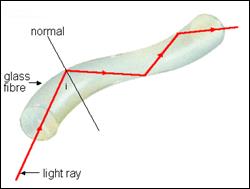
Applications of Total Internal Reflection (National 5)
Fibre Optics
‘Cat’s Eye’ retro-reflectors
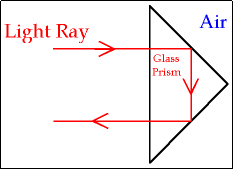
Periscopes
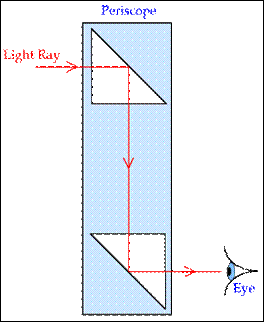
Section 2 – Radiation
Electromagnetic Spectrum (National 4)
(National 5)
Radiation (National 4)
Natural Sources |
Artificial Sources |
Cosmic radiation (Sun, outer space) |
Nuclear power stations |
Radiation from the Earth’s crust |
Nuclear medicine ( X-rays, radiography) |
Radiation from the human body |
Testing nuclear weapons |
Medical |
Industrial |
Destroying cancerous tumours, |
Nuclear power stations |
Diagnosing problems inside the body |
Finding faults in building materials and sources of pollution/leaks |
Sterilising medical equipment |
Smoke alarms |
Radiation (National 5)
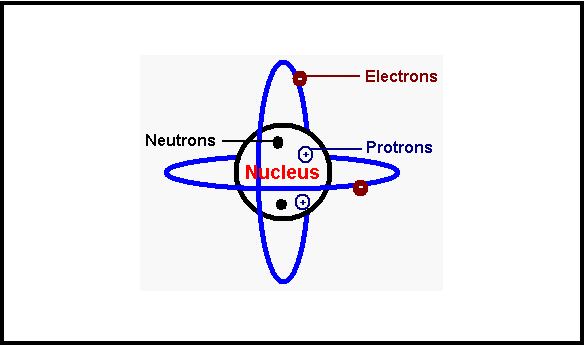
Some elements in the periodic table are radioactive. This is due to the nuclei of the atoms within that element disintegrating and emitting radiation.
Alpha |
a |
2 protons (+ charge) |
Emitted from the nucleus. |
Beta |
b |
An electron ( - charge)
|
Emitted from the nucleus when a neutron changes into a proton, emitting an electron. |
Gamma |
g |
An electromagnetic wave. |
Emitted from the nucleus. |
charged ion is left behind.
Absorbers (National 5)
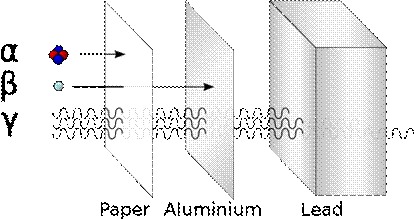
.
Activity of a radioactive source
Activity A = N, number of Disintegrations
(Bq) t, time (s)
Radiation (National 5)
Half – Life
When a radioactive source disintegrates, the activity (number of disintegrations per second) depends only on the number of radioactive nuclei present, so the greater the number of nuclei disintegrating each second, the greater the activity of the source.
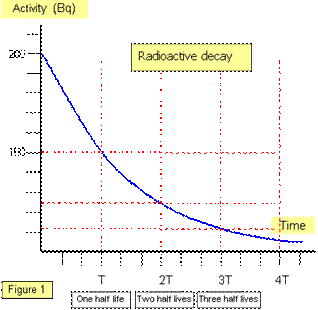
Radiation (National 5)
We can also find the half-life using numerical data:
A radioactive isotope gives an initial count rate of 1800 Bq,
after 120 minutes the activity is 225 Bq, calculate the half-life.
Activity (Bq) |
1800 |
900 |
450 |
225 |
Time (mins) |
0 |
T1/2 |
T1/2 |
T1/2 |
3 half-lives (T1/2) = 120 minutes, therefore T1/2 = 40 minutes.
Absorbed Dose (D)
Absorbed Dose = Energy absorbed ÷ mass of absorbing material
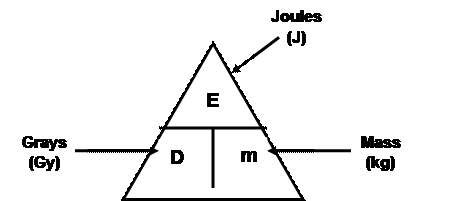
Equivalent Dose (H) (National 5)
Radiation |
Radiation Weighting factor (wr) |
Alpha a |
20 |
Beta b |
1 |
Gamma g |
1 |
Fast neutrons |
10 |
Equivalent Dose (H)
To calculate the Equivalent Dose when a person is exposed to more than one type of radiation, we calculate the equivalent dose for each type of radiation using the above equation and add them all together.
Example (National 5)
A worker is exposed to:
Calculate the total Equivalent Dose received by the worker.
Gamma : H = DWr
Total H = (15 + 8 + 0.6) x 10-3 Sv
= 23.6mSv
= 0.0236Sv
Alpha : H = DWr
H = 400 x 10-6 x 20
= 8 x 10-3 Sv
Beta : H = DWr
H = 600 x 10-6 x 1
H = 0.6 x 10-3 Sv
Radiation (National 5)
Fission
In a fission reaction, a large unstable nucleus splits into two smaller nuclei with the release of several protons and energy.
E = mc2
E = energy released (J)
m = ‘lost mass’ (kg)
c = speed of light ( 300,000,000m/s)
Radiation (National 5)
Fission
Radiation (National 5)
Fusion
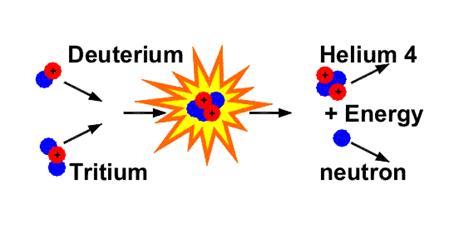
In a fusion reaction, two light nuclei combine to form one larger nucleus and energy is given out in the process.
E = mc2
E = energy given out (J)
m = ‘lost mass’ (kg)
c = speed of light (300,000,000 m/s)
Deuterium and Tritium are isotopes of Hydrogen. During fusion, they form a nucleus of Helium and a neutron.
The two smaller nuclei need to collide at very high speeds for fusion to occur. In practice this means raising the temperature of Hydrogen gas to a staggering 100 million degrees Celsius. This happens naturally in our sun.
Scientists have found this reaction very difficult to replicate on Earth due to the high temperatures needed. If we could harness the energy from fusion the world’s energy worries would be over.
10 grams of Deuterium, which can be extracted from 500 litres of water and 15g of Tritium produced from 30g of Lithium, would produce enough fuel for the lifetime electricity needs of an average person in an industrialized country.
Radiation (National 5)
|
Nuclear Fission |
Nuclear Fusion |
Natural occurrence |
Fission reaction does not normally occur in nature. |
Fusion occurs in stars, such as the sun. |
By products of the reaction: |
Fission produces many highly radioactive particles. |
Few radioactive particles are produced by fusion reaction. |
Energy ratios: |
The energy released by fission is a million times greater than that released in thermal chemical reactions; but lower than the energy released by nuclear fusion. |
The energy released by fusion is three to four times greater than the energy released by fission. |
Conditions: |
Fission reaction occurs inside nuclear piles within a concrete and lead reaction vessel. |
Very high temperatures are required. |
Energy requirement: |
Takes little energy to split two atoms in a fission reaction. |
Extremely high energy is required to bring two or more protons close enough that nuclear forces are overcome and the nuclei fuse together. |
KD May 2012
Source: http://www.kgsorkney.com/uploads/1/4/9/3/14935550/waves__radiation_summary_notes_final.doc
Web site to visit: http://www.kgsorkney.com
Author of the text: indicated on the source document of the above text
If you are the author of the text above and you not agree to share your knowledge for teaching, research, scholarship (for fair use as indicated in the United States copyrigh low) please send us an e-mail and we will remove your text quickly. Fair use is a limitation and exception to the exclusive right granted by copyright law to the author of a creative work. In United States copyright law, fair use is a doctrine that permits limited use of copyrighted material without acquiring permission from the rights holders. Examples of fair use include commentary, search engines, criticism, news reporting, research, teaching, library archiving and scholarship. It provides for the legal, unlicensed citation or incorporation of copyrighted material in another author's work under a four-factor balancing test. (source: http://en.wikipedia.org/wiki/Fair_use)
The information of medicine and health contained in the site are of a general nature and purpose which is purely informative and for this reason may not replace in any case, the council of a doctor or a qualified entity legally to the profession.
The following texts are the property of their respective authors and we thank them for giving us the opportunity to share for free to students, teachers and users of the Web their texts will used only for illustrative educational and scientific purposes only.
All the information in our site are given for nonprofit educational purposes
The information of medicine and health contained in the site are of a general nature and purpose which is purely informative and for this reason may not replace in any case, the council of a doctor or a qualified entity legally to the profession.
www.riassuntini.com